Prove It
Why WinterSet, SummerSet and EcoSet Will Improve Your Dairy Animals Health
Richard Heckmann PhD
One of the major challenges for dairymen is to keep animals protected against diseases especially those involved with the mammary glands. Bovine mastitis, inflammation of the mammary gland, is a disease complex caused mainly by a variety of bacteria invading the mammary gland which occurs in acute, gangrenous, chronic and subclinical forms of inflammation. Animal care, especially of the teats and mammary glands, hygiene and herd management are important factors for control of this dairy animal disease. For the past four decades we have researched methods of preventing mastitis with contributions ranging from milking machine inflations to various teat dips. During our investigations the concept of powdered teat dip was formulated. The common teat dips on the market are formulated in a liquid. Major contributors to mastitis for bacteria invading the milk canals are; Staphylococcus aureus, Streptococcus agalactae, Bacillus subtilis, Pseudomonas aruginosa, Escherichia coli, Mycoplasma and others. Our studies have concentrated on the first four. Some of our studies have used bacteria cultured directly from mammary glands of lactating animals. Entry for the mastitic causing bacteria is through the teat orifice.
During our work on inflations for milking machines we noticed the negative effect liquid teat dips had on dairy animals during the winter months especially in cold northern regions of North America. Early in my career I had worked for a chemical company developing powders for treatment against insects on food crops. That was the catalyst for the powder teat dip.
There are several major concerns or questions for a teat dip:
- Can the formulation kill (bacteriocidal) or slow down growth (bacteriostatic) of the bacteria?
- Will the product help heal lesions, cuts, abrasion on the teat?
- Are products within the formulation harmful for human consumption?
- Will the inert carriers for the product cause damage to the teat surface?
- Is it economically feasible to produce the product?
Based on these questions, we started to formulate powder products. Concurrent with this study we had been investigating a herbal product that had many of the properties we desired for the powder dip. In the laboratory we isolated active products from the herb that were bacteriocidal, had healing properties and a cell proliferant (accelerate cell repair).
A liquid dip was tested from the herb on cows with good results.
Powder Dip
The first formulation in a powder form was based on these ingredients in a powder state with isolated factors from the herb: In a mortar and pestle we mixed ascorbic acid, allantoin, talc, and other inert products. This was tested in the laboratory against 3 pure strains of bacteria. The exact formulation cannot be disclosed at this time.
Procedure:
Pure cultures of the three species of bacteria were prepared in a nutrient broth. Then blood agar plates (BA) were “flooded”, with one of the three cultures (duplicate plates for each baterium). This would generate an intense bacteria growth beyond what a dairy animal would experience. Then one of the formulations we had prepared (duplicates) was added across the diameter of the BA plate (Figure 1). The BA plates were incubated for 24 to 48 hours at room temperature then evaluated for bateriostatic and bacterial properties. The bacteriostatic/bacteriocidal properties were indicated by measuring from the formulation line how much area had been cleared of bacteria. Ten to sixteen measurements were taken for each plate.
Using the procedure we formulated a product for field testing on a dairy herd (BYU Dairy Herd). The product was tried on dairy animals with abrasions and cuts to test healing properties.
Figure 1: Technique for Analyzing and Evaluating Bacteriocidal Properties.
Flooded BA plates
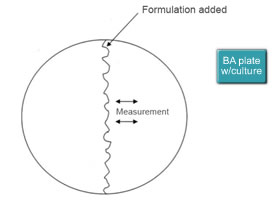
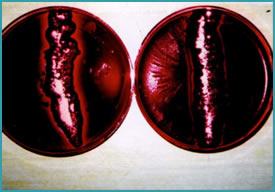
Table 1. Field Trials for Healing, BYU Dairy Herd
| Cow Number | Treatment* | Description | Observation |
| 3584 | B | One crack 0.5 cm LR |
Healed after 2 days of treatment |
| 3158 | B | Three Cracks cm RF 0.5 cm RF 0.3 cm RF |
All three healed after 3 days |
| 3304 | B | Four Cracks 0.5 cm RF 0.8 cm RR 0.3 cm RR 0.2 cm RR |
Healed after 2 days of treatment |
| 3734 | B | Wound RF teat 4.5 X 3 cm |
3 days for wound and crack to heal |
| 3308 | B | Two cracks 1 cm RF 0.5 cm RF |
2 days for cracks to heal |
Overall Summary: The formulation can accelerate healing
*This is a product containing both healing and bacteriocidal products within an inert carrier
Changes in Formulation:
After several months of experimentation we have made changes in the powder formulation to enhance the results based on the 5 questions listed previously.
- Bacteriocidal: Some of the initial ingredients became very expensive and not always available as well as having limited shelf-life. We have tested chemical ingredients that have been used for human medicine and other that are considered food grade. In each case the same procedure was followed with 3 to 4 species of bacteria. Garlic has also been tested for killing properties against bacteria.
- Carrier. Due to the irritating nature of some products used as carriers we have replaced these with others.
- Other additives such as flow agents and binders
Results: For current powder formulations
Table 2. Powder Formulation Trials:
Eight formulations of a powdered teat dip for dairy animals tested against 4 species of bacteria. Measurements in millimeters (mm). Average of 10 measurements, 48 hours.
| Bacteria | Formulations** | |||||||
| A | A1 | B | B1 | AB | C | D | E | |
| Staphylococcus aureus | 12.6* | 10.2 | 4.1 | 6.5 | 2.8 | 4.2 | 11.8 | 4.6 |
| Streptococcus agalactae | 11.8* | 10.1 | 7.5 | 7.0 | 3.2 | 8.8 | 11.4 | 7.8 |
| Psuedomonas aruginosae | 3.1* | 1.1 | 1.0 | 1.1 | 1.0 | 1.0 | 3.0 | 1.0 |
| Bacillus subtilis | 6.1 | 9.2* | 5.5 | 9.1 | 4.1 | 4.1 | 10.6* | 4.0 |
*Most effective bacteriocidal formulation
AB: Product on the Market, currently offered by a competitor
**Due to proprietary nature of the products, letters are used for designation.
The latest tests for the formulation were conducted during March, 2010 on 4 strains of bacteria using a competitors formulation and two formulated by Dairy Health Products, Silicone Products (Millville, Utah), Winterset and Summerset
Table 3:
Results of latest trial (March 2, 2010) against four strains of bacteria. Measurements in millimeters (mm) representing bacteriocidal action. Average of 10 measurements, taken 24 hours after plates were inoculated with bacteria.*
| Bacteria | Formulations | ||
| Competitors | Winterset | Summerset | |
| Staphylococus aureus | 3.7 mm | 5.6 mm | 4.9 mm |
| Stretococcus agalactae | 5.5 mm | 6.5 mm | 5.4 mm |
| Pseudomonas aruginosa | 0.8 mm | 2.6 mm | 1.3 mm |
| Bacillus subtillis | 3.4 mm | 10.0 mm | 7.0 mm |
* We usually measured the bacteria killing action after 48 hours post inoculation.
Comments on Results:
Winterset, Summerset and EcoSet had better bacteria killing properties then the competitors. From Table 2 the best formulations were A and D which are similar to Winterset formulations (see Table 3). Note that the trial for Table 3 was conducted for 24 hours while Table 2 represents a 48 hour trial.
My studies are done “blind” whereby I am not aware of the source or ingredients of the formulation of the product at the beginning of the study. A major criterion is the effect the product has on the Pseudomonads (Pseudomonas aruginosa) which are resistant against most bacteriocidal products and represent a major component for dairy animal health. If you compare (D) against the product generated by a competitor for the market (AB) you will note that measurements for AB (1.0) and D (3.0) for their effect against the pseudomonad (Table 2).
Powder Teat Dips are effective for dairy health especially in the management of mastitis. They can be used during all seasons of the year.
